Syllabus
Catalog description
(Prerequisites - EM121, MA113, PH111) Concepts of system, conservation and accounting of extensive properties, and constitutive relations are introduced as a common framework for engineering analysis and modeling. Conservation equations are developed for mass, charge, momentum, and energy, and an accounting equation is developed for entropy. Applications are taken from all engineering disciplines.
Instructors
 |  | ||
 |  |
Text
Course Notes for ES201 are available for purchase at the Rose-Hulman Book Store:
D. E. Richards, Basic Engineering Science-A Systems, Accounting, and Modeling Approach (Fall 2007 Version)
Additional handouts throughout the course will supplement the notes as required.
⇧Course goal & content
The overall goal of this course is to help you begin to think like an engineer so that you can solve real life engineering problems. Specifically, this course will present a unifying framework for solving engineering problems based upon the engineering science concepts of conservation and accounting principles, properties of matter, and constitutive equations. This framework combined with the appropriate mathematics will enable you to begin developing models that describe the behavior of real engineering systems. Course goals have been prepared for this course and will be distributed separately. In addition, lists with specific objectives will be distributed throughout the quarter to help guide your study and monitor your progress. These lists will serve as one of your best study guides.
Course activities & philosophy
Mastering any new subject requires continuous effort by the learner to make sense of new ideas and concepts and to relate them to what you already know. Learning to identify, formulate, and solve problems requires diligent practice in applying a logical problem solving methodology. Learning to solve real life engineering problems also requires a willingness to deal with ambiguity and uncertainty.
None of the objectives of this course are supported by passively sitting in a classroom, listening to a lecture that repeats the assigned reading, and copying down material you were supposed to have read last night. Throughout this course, we will attempt to make learning an active instead of a passive process. Studies have repeatedly shown that students learn more when they are actively engaged in applying new material. Educational research has also shown that cooperative learning is one of the most productive, although not the only, type of active learning.
Active learning requires that you do things to help you learn, e.g. questioning the material, figuring out how it relates to what you already know, looking for connections, looking for differences, creating a visual outline of the material (concept mapping), outlining, paraphrasing, teaching it to a friend—in short, getting to feel like you "own" the material.
Both individual and cooperative active learning exercises will be used throughout this course. In cooperative learning, you interact with others in a small group to perform a specific task. Working with peers provides immediate feedback and support as you work together to complete the task and understand the material. In addition, working in groups teaches you social skills that will be invaluable as you move into the team oriented world of industry.
This course is organized around two different types of activities. Development activities give you opportunities to develop your skills and improve understanding of the material. They will occur continuously throughout the quarter and are described in more detail in the next section. Evaluation activities give you opportunities to demonstrate to the instructor your understanding of and ability to apply the course material. These will occur four times during the quarter: three midterm exams and a final exam.
⇧Development activities
Homework
The purpose of homework assignments is to give you an opportunity to learn and practice new skills—in short to help you learn new material. Do the homework daily and don't let it pile up. You are strongly encouraged to attempt all homework problems on your own before you discuss the problem or seek help from others. Experience has shown that a significant part of the benefit of doing homework comes from this independent attempt of the homework, i.e. applying your skills and knowledge with a problem statement and a blank sheet of paper. (In hindsight, most solutions look obvious.) Complete solutions for selected problems are available in the student solution manual, available for download in the Files section of the course website.
Readiness assessment tests (RATs)
Everyday you should come to class prepared to take a brief quiz—a Readiness Assessment Test (RAT). A RAT will typically cover the key points of the assigned reading, key ideas from the previous lecture(s), and key concepts and definitions. Sometimes the instructor will indicate specific material for a RAT. RATs will be brief (approximately 5 minutes) and will be given at the beginning of class. Makeup RATs will not be given to latecomers or absentees.
Active learning exercises (ALEs)
Active learning exercises require you to work individually or in groups and will be used frequently in class to help you understand the course material. These activities may or may not be graded. Makeup ALEs will not be given.
On Fridays in Dr. Thom's section, the solution space for this work will consist of small, 2'×2' whiteboards, on which you and your team mates will collaborate. This shared solution space has been shown to enhance greatly the amount of true collaboration during problem solving, and also makes it very easy for the instuctor to give immediate and individual feedback to students. You are welcome to take pictures of your work during class using your cell phone or other smart device.
⇧Course expectations
Work load
This is a four-credit course. The workload for this course should average 8 to 12 hours a week outside of class. This time should be spent reading the text and notes, working problems, discussing material with your colleagues, and thinking about the material in this course.
Reading assignments
You are expected to complete the reading assignments before class. In-class activities assume that you are familiar with the assigned reading material.
Homework assignments
- Homework is assigned daily and each day's homework is treated as a set. A set will typically consist of 2 - 4 problems. Unless you are told otherwise, you should work on homework following the class period in which it is assigned.
- Homework sets will be collected at the beginning of the class period on the days indicated in the schedule.
- Only work submitted by the due date can be accepted, promptly evaluated, and returned. Only medical issues or prior arrangements with the instructor are acceptable reasons for turning work in late.
- Homework is graded mainly on effort in order to encourage you to truly work the problems and learn from the experience, as opposed to copying solutions from friends, campus files, or illegally downloaded solutions manuals. A cover page for homework assignments with a grading rubric is found on the Other files subsection of the Files page. Please print this out and inlcude it with each assignment. (You are welcome to use the back of otherwise scrap paper for this cover page.)
- See detailed Homework Guidelines for more information.
Attendance
Excused absences must be arranged in advance. The instructor reserves the right to reduce your final course grade by one letter grade for every four unexcused absences. Eight or more absences excused or unexcused may result in your failing the course. If you miss class, it is your responsibility to obtain all assignments and handouts from students who were present in class.
Midterm exams
All studentsd are expected to be available for the midterm exams that will be given on each of the following dates. No make-up exams will be given.
Exam 1 - Thursday, Sep 21, 2017
Exam 2 - Thursday, Oct 19, 2017
Exam 3 - Friday, Nov 3, 2017
Final Exam: The final exam will be comprehensive. Every student is expected to be available during the Final Exam Period scheduled by the Registrar.
⇧Course grade components
Final course grades are caculated as given in Table 1.
Table 1: Final grade breakdown| Development activities | |
| Homework, RATs, and other graded activities | 12% |
| Evaluation activities | |
| Midterm exams (3 @ 18% each) | 54% |
| Compresshensive final exam | 34% |
| Total | 100% |
Grading standards
Letter grades in this course are established by comparing your performance against an absolute standard of performance. Typically, 90% and above is an A, 80% and above is a B, and 70% and above is a C, and 60% and above is a D. A performance level below 60% will typically result in a letter grade of F.
You must earn at least a C or better in ES201 before you can take the next two courses in the Sophomore Engineering Curriculum: Fluid Systems (ES202) and Mechanical Systems (ES204). In the judgment of the SEC faculty, at least C-level performance in ES201 is required for satisfactory performance in ES202 and ES204.
Academic honesty
Any act of academic misconduct is grounds for discipline in accordance with the most recent edition of the Rose-Hulman Institute of Technology Academic Rules and Procedures. If in doubt, ASK! The most recent information can be found on the web at http://www.rose-hulman.edu/campus-life/student-services/registrar/rules-and-procedures/index.html. Specific guidance about collaboration and the use of files is found below under Homework Guidelines.
⇧Homework guidelines
Engineering problem solving involves developing and documenting a solution. The ability to solve an engineering problem is worthless if the engineer is unable to communicate the solution to those who need it. In industry, engineering solutions are often archived and used later to troubleshoot a problem, as evidence in a lawsuit, or as the basis for a new design; thus, documenting your thought process is essential. The following guidelines are presented to help you effectively document and communicate both the solution to the problem and the process used to obtain it. The correctness of any solution can only be judged after evaluating the solution process with its underlying assumptions.
Mechanics of preparing solutions
- Format
- Standard engineering problem paper must be used for all homework. (It is also recommended for your class notes.)
- Each homework set consists of a cover page followed by the homework problems arranged in the order they were assigned. Write your name and campus mail box number in the upper right-hand corner and the problem number in the upper left-hand corner of the first page of a problem. Without a campus mail box number, your homework may never make it back to you. (Here is a sample format, which can also be found in Appendix A of the course text.)
- Start each problem on a new page and use only one side of each page. (We will not look on the backside.)
- Multi-page solutions must be stapled together and have a notation on each page that indicates the number of pages, e.g. Page 2 of 3, or 2/3, or 2 of 3, etc. Note that many students err by trying to cram a solution onto a single page. This not only hurts the appearance but also often "cramps" your logic.
- Each solution must be both legible and comprehensible. If you use the computer, e.g. Maple, Mathematica, Excel, or EES, to solve any part of a problem, a printout must be included with the solution to support your result. Computer printouts are supporting documents but are not the solution. Depending on how they are used they must be annotated appropriately so that they make sense in the context of your solution.
- Coherence & correctness
- Your work must be CLEAR and COMPLETE. In particular, note the following:
- Solutions must be prepared using the problem solving format discussed in the notes, i.e. Known, Find, Given Data & Schematic, Analysis, and Comments. Assumptions must be explicitly stated, but may be made as appropriate in the Analysis section.
- As a minimum a schematic diagram depicting the physical system should be present. Sometimes multiple views or details may be helpful. Identify symbols to represent the important physical informa-tion.
- The solution should be worked through with symbols as appropriate. Remember that you are actually developing a mathematical model of the physical system. The equations should be reduced to final form, i.e. the unknown isolated on one side of the equal sign, before substitution of any numbers. This will aid you in understanding the physics of the problem without getting bogged down in units.
Students frequently carry this to extreme producing excessively long expressions. To prevent this, look for logical intermediate solutions to calculate along the way. For example, you may need the mass m of gas in a tank in a long equation but the mass also depends on pressure, volume, temperature, and the ideal gas constant through the relation m1 = (P1V1)/(RT1). Rather than continuing to recopy and manipulate (P1V1)/(RT1), use the symbol m1 for the mass in the larger equation and do a side calcula-tion to compute the numerical value of m1. Then at the appropriate point in the problem insert the nu-merical value. This has the added benefit of giving you multiple places to check the reasonableness of your answers. - Never write the magnitude of a physical quantity without the appropriate units. Experience has shown that failing to carry units along in a calculation is a major cause of mistakes. (That's also why using symbols as long as possible is a good idea.) Numerical answers reported without units are worthless and unacceptable! Unit errors are also easier to check with several intermediate values as mentioned above instead of one long equation.
- State the source of values not given or calculated, e.g. From Table 3.1 in Textbook XYZ.
- Clearly identify intermediate and final answers.
- Check your work. In particular
- check the answer for correct dimensions and units.
- evaluate the reasonableness of the trends and calculated values.
- try to find a "ballpark" estimate to bracket and support the correctness of your answer. (What calculation could your boss perform in thirty seconds on the back of an envelope that checks your results?)
- Miscellaneous
- You are encouraged to attempt every homework problem assigned. Homework problems are selected to illustrate specific points or approaches; thus, failing to attempt a problem may keep you from a key learning experience.
- If you are unsuccessful in solving the problem, but you submit an acceptable partial solution you will re-ceive a substantial part of the credit.
An acceptable partial solution must include the following sections:
Known
Find,
Given Data & Schematic
Analysis: This section documents your solution attempt and must include an explicit statement or question indicating why you are stumped, e.g. what is giving you problems or what's missing that you need to know to proceed?
Getting help with homework
A fundamental principle of academic integrity is that you must fairly represent the source of the intellectual content of work you submit for credit. In this course, collaboration on homework is allowed unless otherwise directed as long as all sources consulted (both literature and people) are acknowledged clearly at the end of each problem. Failure to acknowledge the contributions of others to your homework solution will be considered cheating. Verba-tim copying all or part of a solution that you submit for credit when you have not made any intellectual contribution is considered to be academically dishonest.
If you need help on the homework use the following resources:
- Get help from a classmate, but do not copy their solution. Discussion among students to understand homework problems or to prepare for exams is encouraged. Working in a group on the homework can be an excellent way to learn the material; however, do not get in the habit of letting others do the thinking for you. Do not divide up the problems, but help each other when you get stuck. Remember that every student will be individually ac-countable for the material on the exams.
- Get help from the instructor. Check with your instructor for office hours and availability.
- Try the Learning Center and Percopo Hall. They both have Peer Tutors with experience in the Sophomore Engineering Curriculum
E-mail etiquette
Professors and students have different schedules, especially sleep schedules! And so it is not reasonable to expect an instantaneous reply to an e-mail sent at 2:30 a.m. It is reasonable, however, for all of us to expect each other to check our e-mail regularly. Let us therefore adopt this policy in regards to e-mail communication:
- We all agree to check our e-mail at least once per business day.
- When a response to an e-mail is warranted, allow a one business day turn around time for the reply.
- Please remember that e-mail is a professional form of communication. It is not the same as texting, and you therefore should generally refrain from that type of informality.
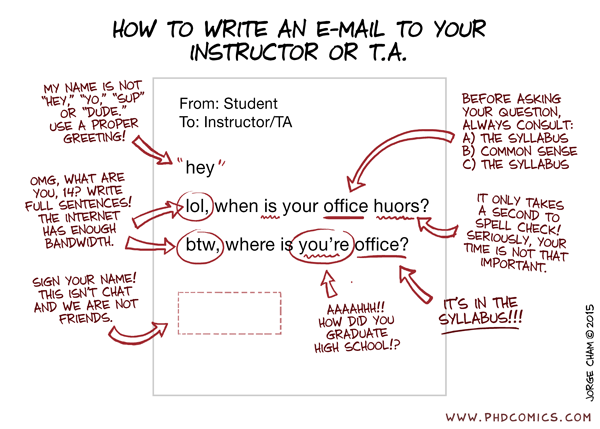
Fig. 1: A snarky example of what makes some professors' blood boil. ⇧
Classroom etiquette
We will do lots of active learning in class! And so, please come each day with
- a writing instrument,
- paper,
- a calculation device,
- and anything else your instructor requests.
I hate to sound like a cranky old curmudgeon, but...
- Do not use your computer in class unless we are doing a computer-based active learning exercise. I.e., no e-mail, Facebook, etc. (Why? Because our brains aren't really capable of multitasking.)
- Same goes for smart phones. (How come? It's the multitasking thing again.)
- Do not work on other class assignments in class. (You guessed it! Multitasking. [sic.])
- Do participate, ask questions and help your neighbors. We're all in this together!
Disclaimer:
The instructor reserves the right to modify course content, schedule, policies, etc. as outlined in this contract.
Please, don't...
All of these things are implied in the contract above, but for the sake of completeness (and from having oberved students do them all too often) I include this list of things you really ought never do. Ever.

Fig. 2: Yoda is a wise soul, indeed. ⇧